 Safety
standard UL, CSA, IEC60601-1 / UL, CSA, IEC60950 compliant! Safety
standard UL, CSA, IEC60601-1 / UL, CSA, IEC60950 compliant! |
| |
No need to prepare extra Fuses for double or reinforced insulation. |
 Fuses
equipped in both L(live) and N(neutral) input line Fuses
equipped in both L(live) and N(neutral) input line |
| |
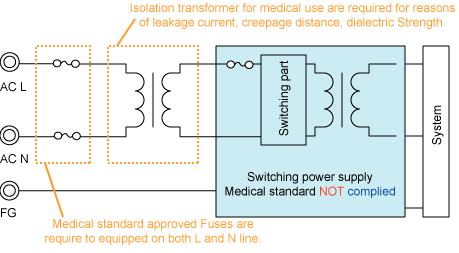
Figure 1. Power supply NOT complied with
Medical standard
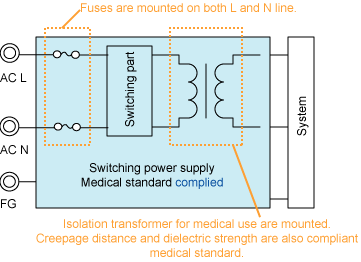
Figure 2. Power supply complied with Medical
standard
|
 Low leakage current Low leakage current |
| |
0.3mA or less (at AC 264V input)
|
 Double and
reinforced
insulation (between
primary and secondary) Double and
reinforced
insulation (between
primary and secondary) |
 Conducted
emission class B compliant Conducted
emission class B compliant |
| |
Some power supplies specifies less leakage current than conducted
emission, but ours satisfy both less leakage and conducted emisson.
|
 Convenient size for loading system rack Convenient size for loading system rack |
| |
Hight 3U, width 1U/2U sized rack embedded size. |
 12V standby power supply output 12V standby power supply output |
| |
0.3A output is possible as auxiliary power (stanby output). |
 RoHS
directive RoHS
directive |
 Blackout detection signal available Blackout detection signal available |
| |
24V output type is available backup operation when connected
to dedicated battery pack |
 Four
categories for Medical equipment Four
categories for Medical equipment |
| |
Medical equipments are classified into 4 different categories
I-IV, based on its severity of effects on human body.
In 2005, amendment of certification system enable private certification
organizations to certify lower risk medical equipment (classII)
on behalf of county against the companies handling medical equipments
and foreign diagnosis drugs. Following shows difference of certifying
divisions between past and after 2005.
Table 1. Difference of certifying divisions
International division |
Medical equipment division based on risk |
Past |
After 2005 |
| Class I |
Effects on human body in case of failure is considered very
low. (Ex. extrasomatic diagnostic instrument, X-ray film) |
Need no certification |
self-certification |
| Class II |
Effects on human body in case of failure is considered lower.
(Ex. MRI, electronic blood pressure, digestive catheter, ultrasonograph) |
Government certification |
Certification by third party |
| Class III |
Effects on human body in case of failure is considered higher.
(Ex. dialyzer, artificial ventilator) |
Government certification |
Government certification |
| Class IV |
Effects on human body in case of failure is considered loss
of life. (Ex. pace maker, artificial heart valve) |
For correspondence of class III and IV, please contact us.
|













